Lately, I’ve been fielding this question frequently from my patients. Before the pandemic, the answer was straightforward, as the flu shot was the sole topic of discussion. However, with the advent of the COVID pandemic, vaccination against COVID became pivotal in protecting us from severe COVID pneumonia. As the SARS-CoV-2 virus evolves and new variants surface, various boosters have been developed and recommended by the CDC. In 2023, the FDA approved two Respiratory Syncytial Virus (RSV) vaccines for adults aged 60 and older, providing more options. Choosing among these vaccines and determining the optimal timing for administration has become a significant concern.
The CDC states that “Getting multiple vaccines at the same time is safe”. This statement is based on the expertise of CDC experts, drawing from indirect evidence and personal experience, as there haven’t been clinical trials studying the safety of combining influenza, RSV, and COVID vaccines. Let’s delve into each vaccine individually before forming your conclusion.
The influenza vaccine is seasonal, recommended for individuals aged 6 months and older. The influenza virus evolves rapidly, altering its genomic structures frequently. To produce the influenza vaccine before the flu season, pharmaceutical companies must predict the predominant strain for the upcoming season. This prediction relies on global flu surveillance data, genetic/antigenic data, and human serology data. However, accuracy in prediction models is a challenge, affecting vaccine effectiveness. It’s not uncommon for the predicted predominant influenza strain to differ from the actual strain, contributing to the fluctuating efficacy of the vaccine year over year. As shown in Figure 1, the vaccine effectiveness against influenza A in the 2022-2023 season was 39% among adults aged 65 and above. While 39% might seem low, it’s a commendable number compared to the 2017-2018 season (Figure 2), where the effectiveness among adults aged 65 and above was only 11%. Thus, don’t be surprised if you still contract the flu despite receiving the shot.
Given the unreliable effectiveness, why should we get the flu shot? According to the CDC, the mortality rate for the flu is 1.8 people per 100,000, a number that rises significantly among those aged 65 and older. The purpose of the flu shot isn’t solely to prevent mild influenza infections but to help your body establish a degree of immune protection, reducing the risk of severe influenza pneumonia and improving survival rates. Multiple studies have demonstrated these benefits (Clin Infect Dis. 2022, 74(8): 1329; Eur Respir J . 2011,38(1):147.). While 30-40% of people may experience some side effects after influenza vaccination, most of these reactions (76.4%) are mild, such as injection site pain (60.3%), muscle aches (34.5%), and overall discomfort (22%). Considering the benefits and risks, taking the shot is worthwhile, even if the effectiveness isn’t ideal—it’s better than nothing.
Fig 1. Vaccine effectiveness against laboratory confirmed influenza A in emergency department/urgent care settings, October 15, 2022-January 24, 2023
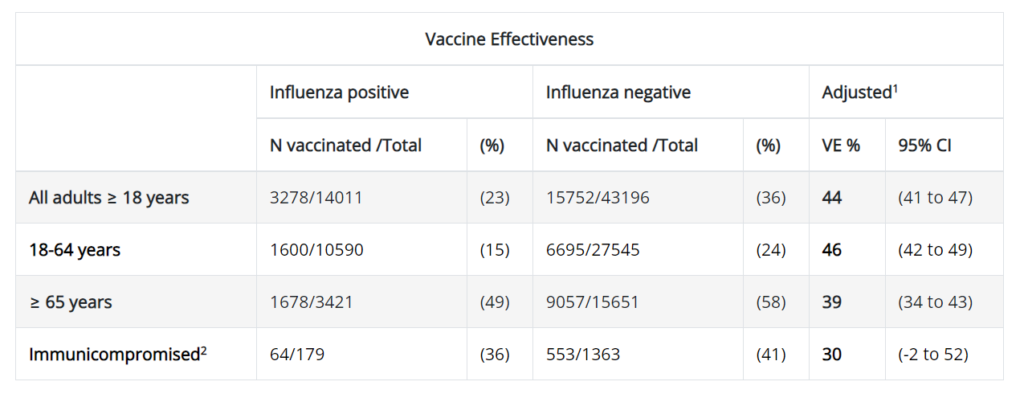
Fig 2. Influenza vaccine effectiveness for all vaccine types, Influenza A viruses, 2017-2018
RSV is a prevalent disease among both children and the elderly, capable of causing severe pneumonia. Currently, there is no antiviral medication available for RSV. The FDA has approved two RSV vaccines for adults aged 60 and above. In a clinical trial (N Engl J Med 2023;388:595), the RSV vaccine Arexvy by GSK demonstrated an efficacy of 82.6% against all types of RSV infection. It proved even more effective in preventing severe diseases, with an efficacy of 94.1%. Another RSV vaccine, Abrysvo by Pfizer, is also effective in preventing lower respiratory tract illness, with reported efficacy at 66.7% (N Engl J Med 2023;388:1465). However, in the Arexvy (GSK) study, the overall adverse event rate and severe adverse event rate were 33% and 4.2%, respectively, while in the Abrysvo (Pfizer) study, these rates were 9% and 2.3%, respectively. Notably, individuals with certain medical conditions, such as active cancer and a weakened immune system (immunodeficiency or taking immunosuppressants), were not included in these trials, leaving the effectiveness in these populations uncertain. The key takeaway is that both RSV vaccines are effective in preventing RSV infection in adults aged 60 and above, but Arexvy has a higher efficacy with more frequent adverse effects.
Shifting to the COVID vaccine, the recommendation remains consistent over the years. Believers may not need additional data, while deniers may remain unconvinced. In general, I recommend my patients to receive the COVID booster.
Although the CDC states that it is safe to receive the three vaccines (flu, RSV, and COVID) simultaneously, I advise my patient not to do so, due to the lack of direct evidence supporting this practice. Each individual vaccine may cause mild discomfort due to the immune reaction it induces. Administering them concurrently might amplify this immune reaction, resulting in unbearable discomfort. This could lead to uncertainty for patients regarding the cause of discomfort, discouraging them from future vaccinations.
Furthermore, from an immunology standpoint, considering the ongoing COVID pandemic since 2020, most individuals have either had a COVID infection or received COVID vaccines, establishing a certain degree of immune defense against SARS-CoV-2. While new variants emerge annually, without a booster, the existing immune defense may not specifically target the new variant but remains protective. Hence, I recommend patients aged 60 and above to first receive a flu shot, followed by the RSV vaccine one or two weeks later. I advise them to receive a COVID boost; however, the decision on whether to receive one depends on their prior experiences, especially for those who had a challenging experience after the original booster.